The Single Swap Rule – Chemistry Short Tricks
We don’t think there are that many “tricks” to doing well in organic chemistry, but there are a few. We was kind of surprised last month when We talked to a number of students who hadn’t ever come across today’s trick, which is probably the most useful of them all. We used to call it the “R/S toggle” but a much better name for it comes from Steven, who calls it the Single Swap Rule.
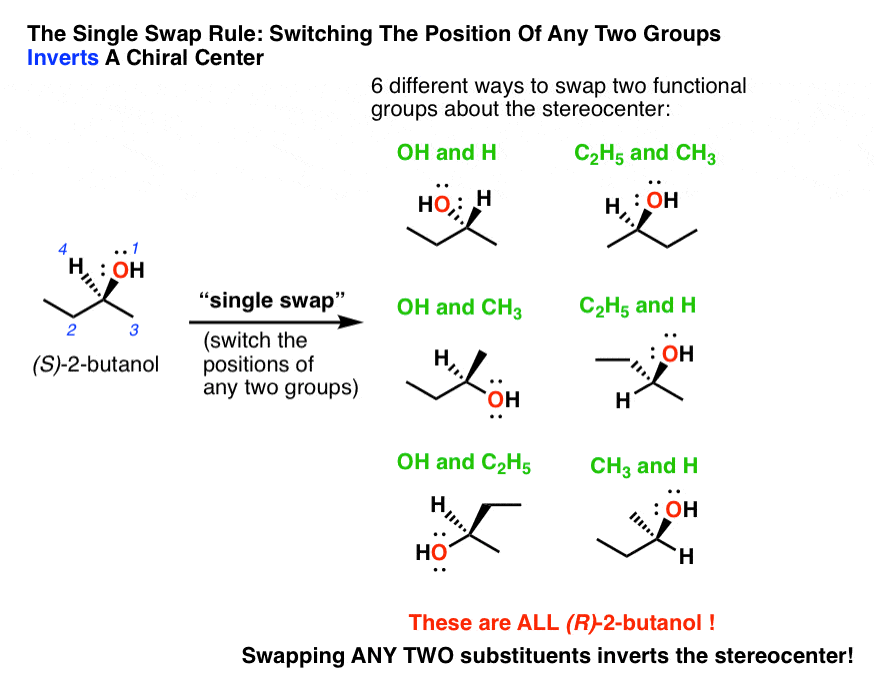
Here’s how it works. Take a molecule with a stereo-center, like (S)-2-butanol (drawn below). If you swap ANY TWO substituents, you will invert the configuration of the stereo-center. That is, switching any two substituents will give you (R)-2-butanol.
There are six possible ways you could do this, and they’re all depicted below.
By the way, don’t take my word for it – if you can’t see this, demonstrate to yourself that each of these six molecules is (R)-2-butanol .
The Single Swap Rule comes in handy for two situations in particular.
- Determining whether a stereo-center is (R) or (S)
- When you need to draw the enantiomer of a stereocenter – like when you are drawing the product of an SN2 reaction for instance.
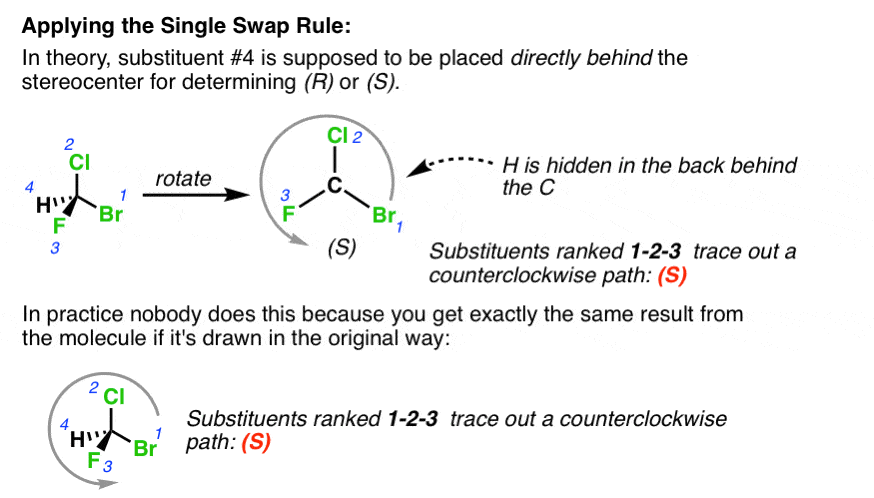
The first order of business in determining whether a stereocenter is (R) or (S) is to number the priority of the substituents according to their priorities. I’m not going to talk about how to do this today, although there are plenty of great resources on how to do so if you’d like to practice this essential skill. According to the rules, once you’ve ordered the substituents, you need to put the 4th ranked substituent in the back and then determine whether the 1st, 2nd, and 3rd ranked substituents trace a clockwise or counterclockwise path. In practice, few people follow this to the letter. That’s because you still get the same result if the 4th ranked substituent is connected to a dashed bond.
The upshot of this is that determining (R)/(S) is fairly straightforward as long as the 4th ranked substituent is dashed. But what if your examiner unhelpfully draws the 4th ranked substituent as a wedge, or even off to one side?
That’s where the Single Swap rule comes in handy.
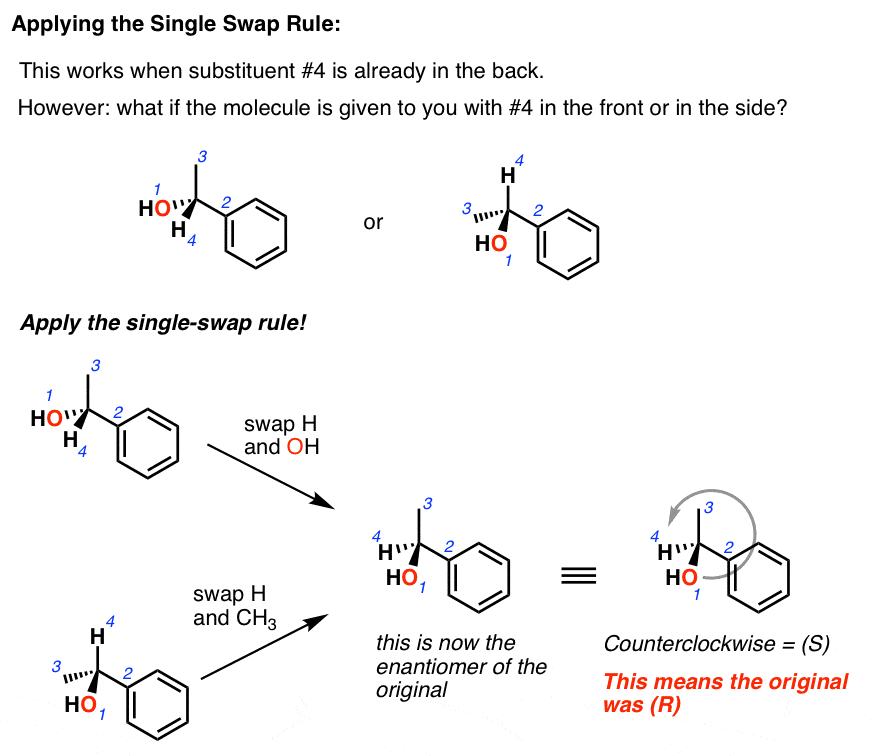
Let’s say we’re given this molecule. Note that priority #4 is pointing out in front. Instead of redrawing the whole molecule to depict it such that #4 is in the back, we can now bust out this trick. Switch the #4 substituent with whatever is in the back. Since we’re doing a single swap, we’re going to *invert* the stereo-chemistry from (R) to (S) or vice versa. Now since the #4 is in the back, we can easily determine (R)/(S) – and here, it’s (R). Since we did a single swap, that means that our original stereo-center must have been (S).
What if #4 is pointing off to the side? Same thing. Swap it with whatever in the back. This will invert the stereo-chemistry. So again, when we determine (R)/(S), we remember that our original stereo-center will be the opposite.
Drawing the enantiomeric configuration at a stereocenter.
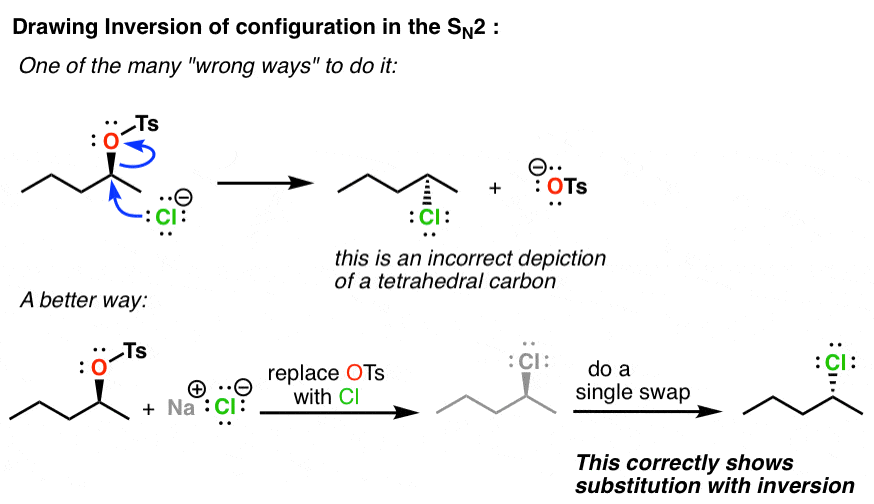
The Single Swap Rule also comes in handy when you need to show that a stereocenter has gone through inversion of configuration. If you’ve studied the SN2 reaction, you know that due to the backside attack, this reaction proceeds with inversion. But I’ve seen lots of students get confused when it comes time to actually draw the products. The single-swap rule makes it easier. Here’s an example of what not to do – and also a way to systematically apply the Single Swap rule in order to be able to reliably draw inversion of configuration.
Do this; 1) draw your starting material. 2) do a single swap. this will invert the stereocenter. 3) replace your leaving group with your nucleophile. That’s it. You’ve now shown an SN2 with inversion of configuration.
[Note: All SN2 reactions occur with inversion of configuration. However, not all SN2 reactions convert (R) to (S) (or vice versa) because priorities of the nucleophile and leaving group can be vastly different. The Single Swap will accurately show you the configuration, but you should determine the R/S of the SN2 product for yourself]
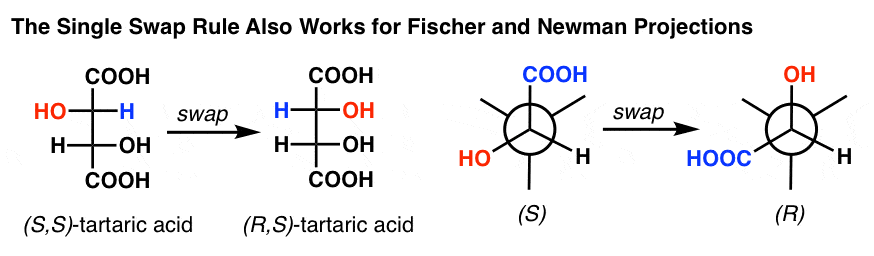
The Single Swap also works for Fischer projections, Newman’s, you name it – this is why, I hasten to note, you aren’t allowed to flip any two atoms on a Fischer – you end up getting the opposite configuration.
With practice you’ll end up doing this in your head – just figure out the priorities, trace the path of 1,2, and 3, and if #4 is in the front or on the side, remember that it’s the opposite of whatever you get (because #4 is in the front). It’s probably the most useful “trick” there is in Org 1, and we still use it almost every single day.